How to freeze dry faster

Most commonly known as freeze drying, lyophilization is a dehydration process used to preserve a perishable or biological material. Freeze drying drastically reduces the surrounding pressure around a frozen sample, allowing the frozen and bound water in the sample to sublimate directly from the solid phase to the gas phase. The water vapor is collected on a condenser that is ideally 15°C to 20°C colder than the freezing point of a sample.
The lyophilization process is slow and can take days depending upon the samples and the freeze drying conditions. These simple steps can be taken to reduce the required time to freeze dry samples.
Choose a freeze dryer that allows heat to be added to the sample.

The driving force in the lyophilization process is energy in the form of heat. In order for a molecule to undergo a phase change (solid to gas), energy must be provided. The more energy transferred to the sample, the faster the process.
However the amount of energy that can be added is limited by the sample’s eutectic point, which is derived from the part of the sample mixture that has the lowest freezing point.
The most common accessories that provide heat to samples when freeze drying are heated product shelves. The product shelves of most Tray Dryers are microprocessor controlled, and the shelf temperatures are automatically changed according to a program that has been set and stored. Heated product shelves are commonly used with Clear Drying Chambers.
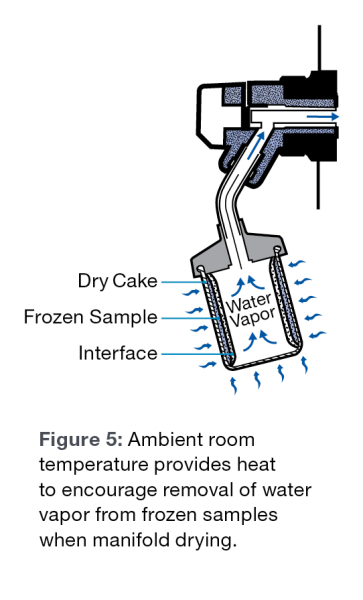
Many end users do not realize that when freeze drying with flasks and manifolds, heat still enters the samples. The heat source is essentially the room temperature that is usually around 20°C to 25°C. Increasing the amount of the sample that is in contact with the glass sides of the flask, will increase the heat transferred to the sample and will result in a faster lyophilization rate. Room temperature variances will certainly affect the required length of time to freeze dry.
The longest freeze dry runs will occur when the sample is placed on unheated product shelves within a chamber or placed inside the freeze dryer’s condenser. It is very difficult to transfer heat through an air gap and even more difficult when the air gap is under vacuum.
 Know the eutectic point of your sample and monitor its sample temperature.
Know the eutectic point of your sample and monitor its sample temperature.
It is critical that a sample remains completely frozen during all stages of freeze drying. The eutectic temperature (triple point) is the temperature at which your sample only exists in the solid phase. If your frozen sample goes to a temperature higher than the eutectic temperature, it will begin to melt.
During the primary drying phase (first phase) of freezing drying heat can be added to your sample to sublimate the water out of the sample. A temperature probe placed in your sample provides feedback of the sample temperature. The more heat you apply the faster the process, however your sample must remain below its eutectic point during primary drying. Knowing the temperature your sample cannot go above enables the greatest amount of heat to be added to the sample without experiencing melt back.
If you do not know the eutectic temperature, it is best to add heat slowly and observe the sample as you gradually add more heat to each subsequent run until you determine the maximum amount of heat that can be added safely to the sample. If the eutectic temperature is unknown, developing the optimal method will require many trial and error runs until the critical temperature is determined.
It is difficult to control the heat that is transferred to the sample when lyophilizing in flasks on a manifold. The heat is essentially the room temperature. If a sample in a flask is melting back (getting too much heat) simply, insulate the flask with foam to slow the heat transfer down.
Putting a flask inside another larger flask with an air gap in between the two flasks is another way to provide insulation to limit the amount of heat that is transferred to the sample.
Perfect your pre-freeze step
How a sample is frozen will not only affect the quality of the final freeze dried sample, it will also affect the length of the lyophilization process.
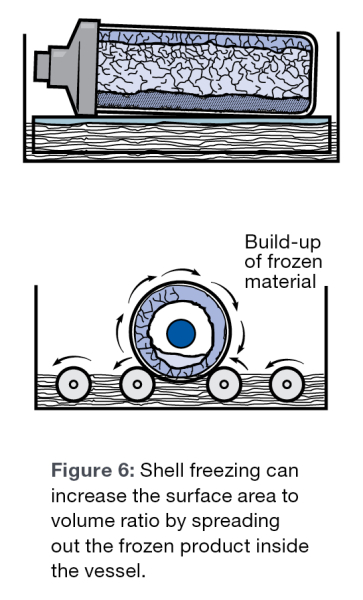
Spreading the sample out to increase the surface area as well as forming a thin layer of ice will speed the sublimation process. Pre-freezing in a thin layer covering the flask is called shell freezing. Commercial shell freezers are available to freeze samples in flasks. By increasing the surface area, more sample is in contact with the flask resulting in better heat transfer to the sample.
As the lyophilization process proceeds, an ice front moves through the sample. This ice front generally moves from the top of the sample to the bottom. As the front moves through the sample, the sublimating water vapor must pass through the dry cake that is formed behind the ice front, as it is being evacuated from the sample to the condenser. The less cake the water vapor must pass through, the faster the molecules will sublimate, resulting in a faster run.
If a shell freezer is not available, it is recommended to slant freeze samples in flasks to increase surface area as much as possible.
The freezing rate of a sample affects the size of the ice crystals that form. Faster freezing rates can cause smaller ice crystals to form. Small crystals result in longer sublimation rates. The cakes produced from samples frozen with large ice crystals are more porous. Water molecules sublimate more easily through porous cakes. The goal of pre-freezing is to form large crystals with a slower freezing rate while making sure the sample does not separate in the longer pre-freeze process.
Set your vacuum level
Sublimation requires energy in the form of heat to be transferred to the sample. Molecules present around the sample help transfer the heat to the sample. If there are no molecules present around a sample, it is difficult to effectively transfer heat. The fastest evaporation rates occur at a vacuum level of .200 mbar.
At vacuum levels lower than .200 mbar, there are not enough molecules present in the system for good heat transfer. At vacuum levels above .200 mbar, there are too many molecules present and this will slow down the sublimation rate.
It is a misconception that the deeper the vacuum level, the faster the sublimation rate. Set the vacuum level in the freeze dry system at .200 mbar to maximize the sublimation rate. If the vacuum level is not set, a deeper vacuum is established and the lyophilization run will take longer.
Know when the lyophilization process is complete
After all of the free water molecules are sublimated in primary drying, secondary drying begins. In secondary drying, the water molecules bound to the sample molecules are sublimated. Heat is required to break the water bond to the sample molecules. Because the majority of the water in the sample has already been sublimated, the sample temperature can rise above the eutectic point without melting back.
It is difficult to know by looking at a sample when the sublimation process is complete. Because ending a run too early can cause a sample to melt back or result in a collapsed cake, the tendency is to error on the side of caution and extend the lyophilization run further than necessary. If the end point can be detected, it can save many unnecessary hours.
While sublimation occurs, the sample undergoes evaporative cooling. Because of evaporative cooling, the sample temperature will be lower than the shelf temperature. With sample temperature probes and heated shelf temperature probes, end point can be detected when the sample temperature becomes equal to the shelf temperature. When these temperatures are equal, sublimation is no longer occurring.
New end point detection accessories are available on some models of freeze dryers to determine the end of the sublimation process for samples in flasks.
By better understanding the lyophilization process and employing these suggestions, hours or even days can be saved when freeze drying.
| chevron_left | Stay safe while processing evidence | Articles | SteamScrubber or FlaskScrubber - That is the question... | chevron_right |