An inexact science: Biosafety risk assessment

In November of 2001, a New York woman, age 57, showed signs of malaise, vomiting, headache, and fever. Five weeks later her illness was identified as an infection of Brucella, caused by a clinical sample processed in the lab where she worked without proper precautions (Noviello, et al., 2004).
A Chicago man was evaluated in September 2009 with fever, body aches, and a cough. Three days later an ambulance delivered him to a local emergency room where he was admitted for worsening symptoms. Less than a day later he died. Over the next week it was determined and reported that the man had died from an attenuated strain of Yersinia pestis – the microbe responsible for the plague (Ritger, MD, et al., 2011).
In both of these instances, it was found that laboratory workers were at risk and succumbed to infections with the very hazards they were tasked with manipulating. Risk Assessments and the controls they promote seek to reduce the possibility of such Laboratory Acquired Infections (LAIs).
Performing risk assessments is one a biosafety officer or laboratory manager’s most vital functions. This multistep process forms the backbone of a microbiology laboratory’s Biosafety Plan, Operating Procedures, and if done early enough, even facility design.
Due to the very nature of work in laboratories, new variables are continually being introduced. This makes any static plan ineffective. To maintain superior and continued safety policies, risk assessments and biosafety plans should be dynamic, living programs; not just mundane exercises.
Risk Assessment
The Process
In terms of analyzing a laboratory’s practices, the purpose of this process is to predict the likelihood of adverse effects that may result from any exposure to health hazards, involving hazard identification and control. Any Risk Assessment can be boiled down to a 5 step process.
- Identify all potential hazards
- Based on likelihood and consequences, prioritize the risk
- Decide how best to reduce risk to an acceptable level
- Design and implement the plan
- Continually evaluate the plan.
Dynamic Interaction
The 5 P’s
Pathogen, Personnel, Place, PPE and Procedures; these are the five facets that are paramount when performing a risk assessment analysis. Each aspect interacts with the other to determine the level or risk and the controls needed to diminish health hazards.
Understanding these dynamics and their interconnectedness is the heart of an accurate assessment for a biosafety officer. “Biosafety is an inexact science, and the interacting system of agents and activities and the people performing them are constantly changing,” (Fleming & Hunt, 2006).
Risk Assessment Triad
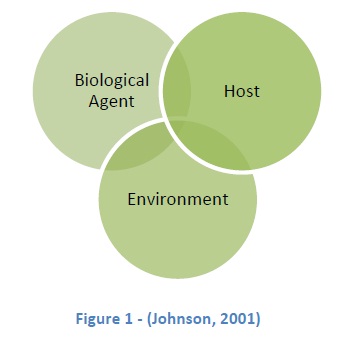
As already stated, risk assessment is not based solely on “measureable, scientific parameters,” yet there are three basic components (Figure 1) making up the basic biosafety risk assessment triad; Biological Agent, Host & Environment (Johnson, 2001). However, each of these mechanisms is made up of its own dependent variables.
Develop a Plan
Biological Agent
When analyzing the risks associated with biological agents, there are multi-level facets to consider. These aspects include:
- Pathogenicity
- Virulence
- Stability in the environment
- Infectious dose
- Route of transmission
- Resistance to prophylaxis
- Availability of prophylaxis
- Host range
- Communicability
- Volume & Concentration
- Type of Manipulation (Procedures)
The purpose is to identify and understand the laboratory’s key biological assets and resources. With this information, the Biosafety Officer can begin to evaluate the risks associated with each hazard.
Host/Personnel
Once the risks are identified and prioritized, a clear understanding of the lab’s human resources must be established. This part of the analysis falls into three main categories; Individual Susceptibility, Managerial Controls, and Occupational Controls.
Managerial & Occupational Controls are typically handled at an executive level, long before a Biosafety Officer begins the risk assessment, (or can be mandated by local, state or federal guidelines). Individual Susceptibility, on the other hand, is wrought with variability, and the most common dynamic to break down. Two factors exist:
- Personal Risk Factors – such as medical conditions/status, age, training, fatigue, immunizations, education, experience, etc…
- Psychological Risk Factors – including perception of risk & safety, taking shortcuts, complacency, habits, etc...
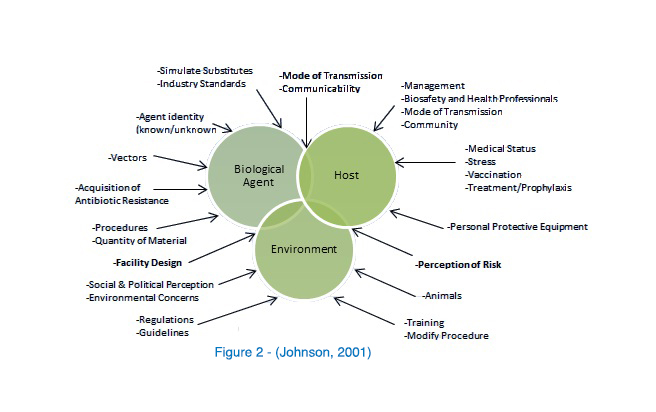
Environment
Identifying hazards and understanding the consequences of the risks is the easy part. What remains is the establishment and implementation of controls to mitigate those risks. This can be free-form; it can be examined from the inside out (starting with the Laboratory itself, moving to adjacent rooms, access corridors, the building and then the community, or vice versa). In any case, the environmental analysis should be performed systematically and then peer reviewed. It is at this stage that engineering controls (safety equipment & facility design), administrative controls (training, SOPs, signage, etc…), and PPE are laid out and selected.
Lastly, execute the plan.
Keeping the Plan Alive
The final step of the Risk Assessment process is never ending; evaluation. Like any good quality assurance and quality control program, the process should be monitored, deviations recorded, and investigations performed with attention to root cause analysis (RCA), culminating in corrective actions. Periodically auditing the plan and processes can proactively identify gaps in coverage and improve the system, possibly preventing a deviation or occurrence. “Observations of individual safety practices, operability of safety equipment, and compliance with safety rules should be part of the audit,” (Adelberg, et al., 1989).
Conclusion
The fundamental core of any lab’s safety program begins with the risk assessment, and ends with worker accountability. No formal process exists for biosafety risk assessment, yet any systematic approach focuses on the same traits and is based on the same premise. Nor is risk assessment a “black & white” objective proposition, it often requires personal judgment based on human, cultural and forecasted factors.
Despite these barriers, a meticulously vetted biosafety plan, through this process, has the capacity to render the most hazardous processes and agents “relatively” safe.
CONTACT A BIOSAFETY APPLICATION EXPERT

Reference and Resource Tools on this subject are provided by USAMRIID, NIH, CDC, & WHO. Special thanks to Tim Sturgis, RBP of the University of Missouri-Kansas City.
Works Cited
Adelberg, E. A. et al., 1989. Biosafety in the Laboratory: Prudent Practices for the Handling and Disposal of Infectious Materials. Washington, D.C.: National Academy Press.
CDC, 2009. Biosafety in Microbiological and Biomedical Laboratories. 5th ed. Washington, D.C.: National Instute of Health.
Fleming, D. O. & Hunt, D. L. eds., 2006. Biological Safety: Principles and Practices, 4th Ed.. Washington, DC: American Society for Microbiology.
Johnson, B. P., 2001. Understanding, Assessing, and Communicating topics Related to Risk in Biomedical Research Facilities. In: Anthology of Biosafety IV - Issues in Public Health. s.l.:ABSA.
Noviello, S. et al., 2004. Laboratory-acquired Brucellosis. Emerging Infectious Diseases, October.10(10).
Ritger, MD, K. et al., 2011. Fatal Laboratory-Acquired Infection with an Attenuated Yersinia pestis Strain. Morbidity and Mortality Weekly Report, 25 February, 60(07), pp. 201-205.
©2019 Labconco Corporation
| chevron_left | How to select the right hood or sample prep equipment for your application | Articles | How To: Protecting Clinical Researchers & Testing Analysts | chevron_right |





