Tuberculosis, Containment Equipment & Laboratories
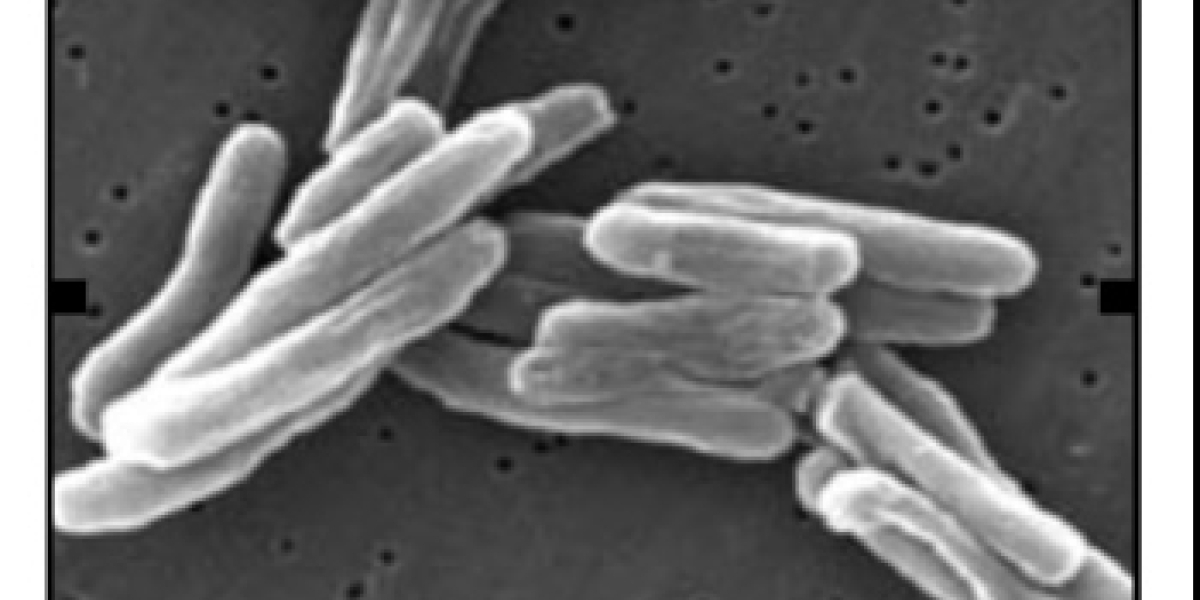
The respiratory infectious disease once known as ‘consumption’ is an infection caused by the bacterial complex tubercle bacilli - TB (tuberculosis forming members of the genus Mycobacterium).
Highly contagious with an infective dose of less than 10 bacilli, Tuberculoses has an estimated 20 million cases world wide. This represents fewer than 10% of the total TB carriers. There were hopes to eradicate tuberculosis; however, drug resistant strains began to show up in the 1980’s giving rise to a new ‘tier’ of TB, Multi-drug resistant TB (MDR TB).
Representing a greater threat and risk level, (MDR) TB is listed in the CDC’s Biosafety in Microbiological and Biomedical Laboratories (BMBL), 5th ed. Section 8 of this document is dedicated to ‘Agent Summary Statements’, where the CDC outlines the general characteristics of the pathogen and its biosafety and biosecurity recommendations.
Contrary to common discussions, TB is only listed as a Biological Safety Level (BSL)-2 agent; requiring BSL-2 level containment, equipment, and practices. Below is an excerpt from BMBL on TB: Laboratory Safety and Containment Recommendations:
BSL-2 practices and procedures, containment equipment, and facilities are required for non-aerosol-producing manipulations of clinical specimens such as preparation of acid-fast smears. All aerosol-generating activities must be conducted in a BSC. Use of a slide-warming tray, rather than a flame, is recommended for fixation of slides. Liquifaction and concentration of sputa for acid-fast staining may be conducted safely on the open bench by first treating the specimen in a BSC with an equal volume of 5% sodium hypochlorite solution (undiluted household bleach) and waiting 15 minutes before processing.
The significance of this statement will be made clear in a moment, but it is important now to point out that it includes not only BSL recommendations, but specific practices to be used and included in SOP generation when working with known or unknown TB containing samples while performing specific tasks (such as AFB and flaming).
That being said, TB and MDR TB can also be used in labs with protocols requiring BSL-3 recommendations:
BSL-3 practices, containment equipment, and facilities are required for laboratory activities in the propagation and manipulation of cultures of any of the subspecies of the M. tuberculosis complex and for animal studies using experimentally or naturally infected NHP . Animal studies using guinea pigs or mice can be conducted at ABSL -2. BSL-3 practices should include the use of respiratory protection and the implementation of specific procedures and use of specialized equipment to prevent and contain aerosols. Disinfectants proven to be tuberculocidal should be used…
The CDC has also released an “Interim Laboratory Biosafety Guidance for Extensively Drug-Resistant (XDR) Mycobacterium Tuberculosis strains.” XDR TB is defined as MDR TB that is also resistant to one of three other tuberculocidal drugs; capreomycin, kanamycin and amikacin. This recommendation supersedes the BMBL when working with suspected or known MDR/XDR TB:
“The risk of occupational infection from XDR TB strains has not been shown to differ from that of non-resistant M. tuberculosis strains. As described in the 5th edition of... (BMBL), M. tuberculosis infections are a proven hazard to laboratory personnel as well as others who may be exposed to infectious aerosols in the laboratory, autopsy rooms, and other healthcare facilities…”
If you are in doubt, or for further questions concerning these or other recommendations, contact your biological safety officer or the CDC. To satisfy these recommendations a Biological Safety Cabinet (BSC) is required. This typically suggests non-ventilated Class I or Class II A2 devices.
A BSC is used for the capture and control of airborne, hazardous aerosols and particulate for the purpose of personal and environmental protection. Unless hazardous chemicals (toxic or volatile) are being used in concentrations or volumes unsafe for environmental recirculation, then the exhaust does not need to be vented to atmosphere.
Yet, many TB labs, including ‘AFB labs’ utilize total exhaust BSCs, incurring major costs both in the construction and in the lifetime operation of the laboratory. This is driven, primarily, by the stigma surrounding TB by architects and engineers, and enabled by laboratory managers and biosafety officers not being informed and active in the planning process of their facilities. Because of this, a point of contention has arisen regarding the use of externally ducted BSCs to double as HVAC exhaust ports for balancing labs.
This opens a clichéd Pandora’s Box of issues regarding proper installation of Class II A2 canopy and Class II B2 cabinets. But it is important to be aware of and to know how to identify over-engineering when building AFB labs, Hot Labs or dedicated TB facilities. Sometimes the simplest looking solution is not the best or easiest solution.
Again, consult with the CDC, know your options, and always perform a risk assessment. As the owner/operator of the facility, challenge the decisions being made if you are hesitant or do not understand what is being proposed or why!
Risk assessments are the most overlooked and underappreciated practices in proposing and designing laboratory spaces and purchasing equipment. Just like the practice of commissioning a new construction, risk assessment reports have the ability to streamline construction and purchasing of the appropriate protective equipment to be installed in the lab. A misstep here could cost a facility tens or even hundreds of thousands of dollars and can delay occupation significantly.
If questions arise, you should contact reputable equipment manufacturers. Their specialists can point you in the right direction. However, as the sole subject matter experts, you know your applications and operations better than anyone; your company should be the primary source for decisions being made when purchasing safety equipment.
| chevron_left | Using the micro IR for genomic DNA isolation | Articles | Labconco and GSA have a history - about 40 years! | chevron_right |






